Our studies
All of our studies are randomized, double-blind, placebo-controlled and designed according to the rules of Evidence Based Medicine
Human Clinical Trials
In-vitro studies and significant findings
2025
2025.01
HUMAN CLINICAL TRIAL PUBLISHED
2025.03
NORDBIOTIC™: COMPLETE GENOME SEQUENCE
2025.02
NORDBIOTIC™ BI040: COMPLETE GENOME SEQUENCE
2024
2024.10
OSTEO DUO: HUMAN CLINICAL TRIAL PUBLISHED
The Effect of Lacticaseibacillus paracasei LPC100 and Lactiplantibacillus plantarum LP140 on Bone Mineral Density in Postmenopausal Women: A Multicenter, Randomized, Placebo-Controlled Study
2024.01
IMMUNOVIR: HUMAN CLINICAL TRIAL PUBLISHED
The role of nutritional support with probiotics in outpatients with symptomatic acute respiratory tract infections: a multicenter, randomized, double‐blind, placebo controlled dietary study.
2024.11
NORDBIOTIC™ LU150: COMPLETE GENOME SEQUENCE
Complete genome sequence of Limosilactobacillus reuteri LU150, a potential vitamin B12 producer from the NORDBIOTIC collection
2024.11
NORDBIOTIC™ LP140: COMPLETE GENOME SEQUENCE
Complete genome sequence of a potentially probiotic cheese isolate Lactiplantibacillus plantarum LP140 from the NORDBIOTIC collection
2024.08
NORDBIOTIC™ LC130: COMPLETE GENOME SEQUENCE
Whole-genome sequencing and characterization of human fecal isolate Lacticaseibacillus casei LC130 from NORDBIOTIC collection
2024.03
GLUTEN DIGEST: GLUTEN HYDROLYSIS
Reducing immmunoreactivity of gluten peptides by probiotic lactic acid bacteria for dietary management of gluten-related diseases.
2024.02
NORDBIOTIC™ LPC100: COMPLETE GENOME SEQUENCE
Complete genome sequence of the probiotic Lacticaseibacillus paracasei LPC100 strain from NORDBIOTIC™ collection isolated from a human fecal sample.
2024.01
NORDBIOTIC™ STRAIN MIXTURE FOR GLUTEN DIGESTION
Lacticaseibacillus casei LC130 strain, Lacticaseibacillus paracasei LPC100 strain, Streptococcus salivarius ssp. thermophilus ST250 strain – composition reducing the immunoreactivity of gliadin peptides and the use of strains for the production of the composition. Polish patent application no. P.447563
2023
2023.07
GASTRO ONE BC300: HUMAN CLINICAL TRIAL PUBLISHED
The Efficacy and Safety of Single-Strain Probiotic Formulations Containing Bifidobacterium lactis or Bacillus coagulans in Adult Patients with Irritable Bowel Syndrome—A Randomized Double-Blind
Placebo-Controlled Three-Arm Interventional Trial.
2023.07
GASTRO ONE BI040: HUMAN CLINICAL TRIAL PUBLISHED
The Efficacy and Safety of Single-Strain Probiotic Formulations Containing Bifidobacterium lactis or Bacillus coagulans in Adult Patients with Irritable Bowel Syndrome—A Randomized Double-Blind
Placebo-Controlled Three-Arm Interventional Trial.
2023.10
SCIENTIFIC RATIONAL OF STRAIN SELECTION FOR CHILDREN’S HEATH
The effectiveness of selected Lactobacillus and Bifidobacterium strains for support the holistic health and well-being of children.
2023.05
NORDBIOTIC™ BABY SCIENTIFIC RATIONAL OF STRAIN SELECTION
The effectiveness of selected lactobacillus and Bifidobacterium on infantile colic.
2023.05
CLINICAL RATIONAL – NORDBIOTIC™ 5 STRAIN COMBINATION
The effectiveness of selected Lactobacillus and Bifidobacterium lactis BI040 strains for support the health.
2023.05
FEMAX ENHANCING IRON ABSORPTION
Development of a Multilayer Probiotic Tablet for Enhanced Iron Absorption: Harnessing the Potential of NORDBIOTIC™ LC130, LPC100.
2022
2021.11
RESISTANCE OF NORDBIOTIC™ STRAINS TO SELEN
Selection of NORDBIOTIC™ strains resistant to selenomethionine.
2022.10
TARGET SHIELD NORDBIOTIC™ PROTECTION OF MICROBIOME
Development of specific NORDBIOTIC™ composition of strains for antibiotic therapy.
2021
2021.02
GASTRO IBS-10: HUMAN CLINICAL TRIAL PUBLISHED
The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study
Hierarchy of Research Designs & Levels of Scientific Evidence
Based on ability to control for bias and to demonstrate cause and effect in humans
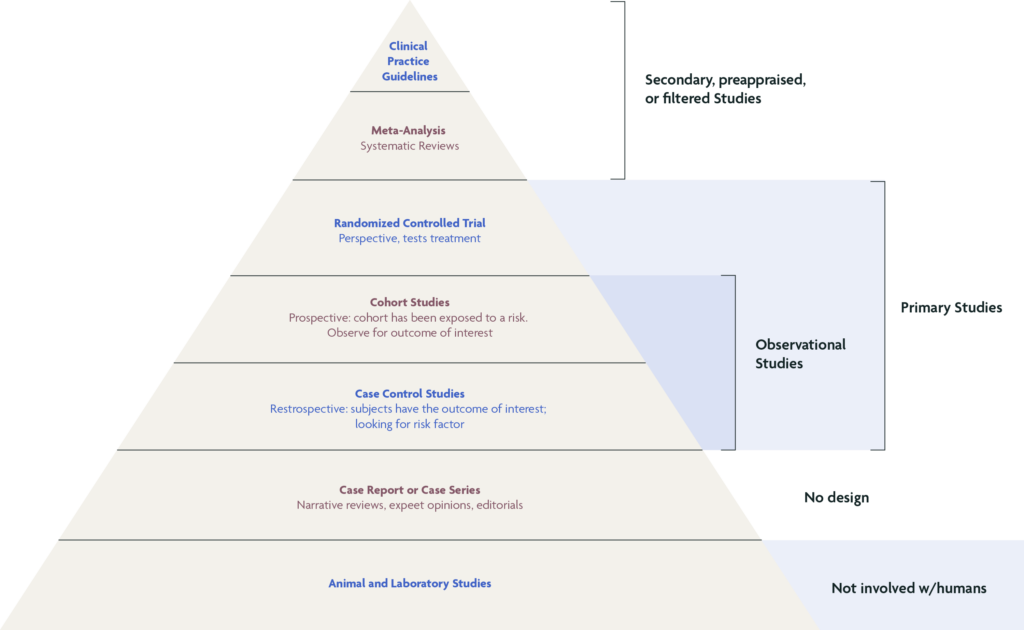
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71‐72.
NORDBIOTIC™ technology research is involved into a range of health areas
Are you a company?
Learn more about our solutions.
Visit nordicbiotic.com